DMF is one of the most widely used solvents in organic synthesis, and is also known as a universal solvent. Today I will share a reaction case with you. The combined use of m-CPBA and DMF is also potentially dangerous. m-CPBA, also known as m-chloroperoxybenzoic acid, is a type of organic oxidant very commonly used in organic chemistry. Relatively speaking, it is relatively safe. However, the field of chemistry is full of unknowns. An accident reported in the literature shared today is related to the mixed use of m-CPBA and DMF, and is directly related to the following chemical transformations.
Synthesizers from Fujisawa Pharmaceutical Company in Japan used m-CPBA to oxidize sulfur to sulfoxide using DMF as a solvent on a pilot scale. The synthesizers first mixed 6.3L DMF and 11.0 kg m-CPBA together, and stirred the two for 2 hours. , Insoluble matter is formed in the system, and then filtered to obtain a clear solution, and the clear solution is added to the organic reaction solution. When the dripping process is carried out for 1 hour, the DMF solution of m-CPBA suddenly rises, and gas is released. , And then suddenly exploded. The author of this article (Org. Proc. Res. Dev.) briefly describes the reaction process as the following figure.
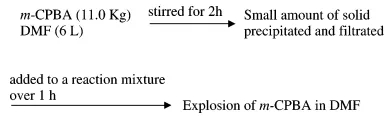
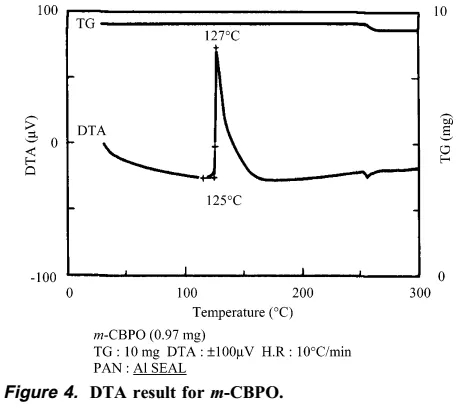
The synthetic personnel immediately searched for the cause of the accident and explosion. They speculated that it was probably caused by insoluble matter, and the insoluble matter was exactly m-CBPO. This insoluble matter may be an impurity from the raw material m-CPBA itself, or it may be gradually generated in the reaction system. The author of this article went on to conduct a series of verification experiments. The author found that the m-CBPO content in the raw material m-CPBA is only 0.2%. In addition, DTA and IST experiments confirmed that m-CPBA melts at 89 degrees and is stable at less than 97 degrees. The author then conducted a DTA study on the DMF solution of m-CPBA, and the results showed that the decomposition temperature of m-CPBA was 83 degrees. The above experiments show that DMF may largely affect the critical point of decomposition temperature of m-CPBA. Therefore, the author believes that DMF solvent played an important role in this accident.

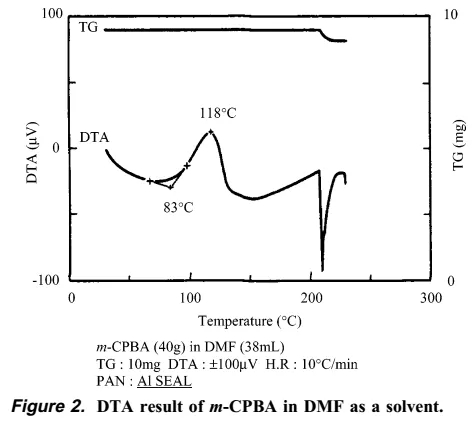
The author then found that as the temperature increased, the content of m-CBPO increased significantly, and DTA research showed that when the temperature reaches more than 125 degrees, a very serious explosion can be foreseen.
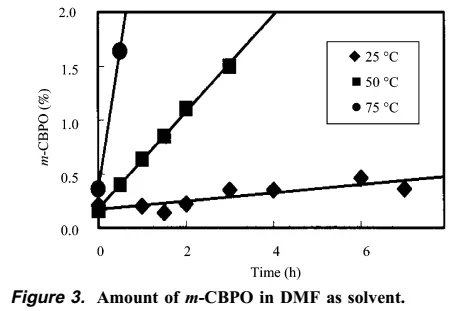
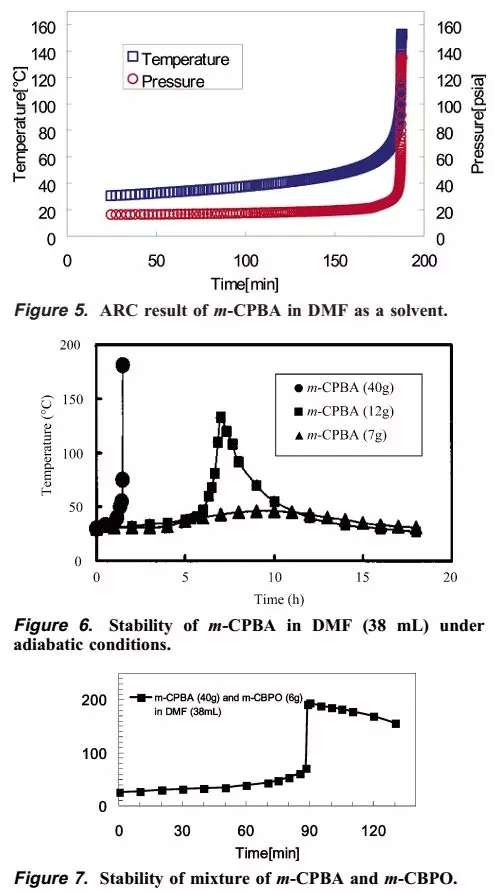
The author then carried out ARC study of m-CPBA DMF solution, concentration study, and mixed stability study of m-CPBA and m-CPBO. The final conclusion is that it takes 185 minutes for the DMF solution of m-CPBA to slowly rise from 26 degrees to 70 degrees, and then it will quickly rise to 200 degrees in a few minutes or the like. In addition, the more concentrated m-CPBA DMF solution heats up faster. The mixed experiment of m-CPBA and m-CPBO shows that the temperature rises slowly at the beginning, but after only 95 minutes, there will be a sharp temperature rise.
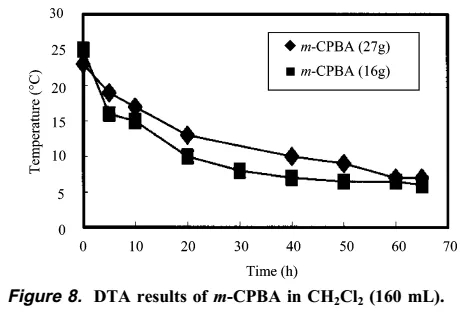
In summary, the author of this article gives the general process of the explosion. First, the formation of m-CPBO leads to an increase in temperature, and then the formation of a large amount of m-CPBO causes an explosion at high temperatures. In the end, the author used dichloromethane DCM as a solvent to successfully solve this problem. DTA experiments show that using dichloromethane as a solvent, there is no exotherm over time.
Experiment with tens of thousands, safety first! DMSO and DMF are strong polar solvents, although they have good solubility for organics, they are also a double-edged sword!
